File:Vaccines-08-00587-g001.webp
From Wikimedia Commons, the free media repository
Jump to navigation
Jump to search
Size of this PNG preview of this WEBP file: 800 × 599 pixels. Other resolutions: 320 × 240 pixels | 640 × 479 pixels | 1,024 × 767 pixels | 1,280 × 958 pixels | 2,560 × 1,917 pixels | 3,539 × 2,650 pixels.
Original file (3,539 × 2,650 pixels, file size: 2.37 MB, MIME type: image/webp)
File information
Structured data
Captions
Captions
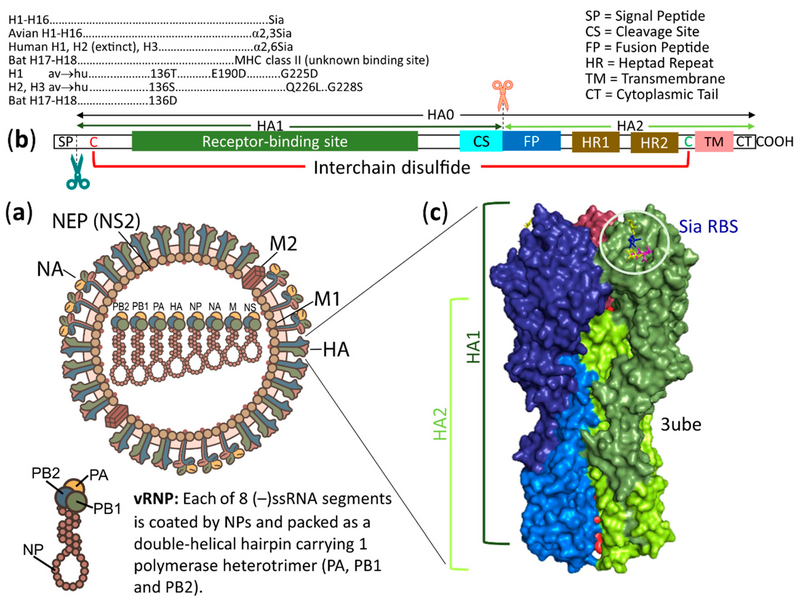
Schematic structures of an influenza A virus (IAV) and its HA.
Summary[edit]
| DescriptionVaccines-08-00587-g001.webp |
English: Schematic structures of an influenza A virus (IAV) and its HA. (a) The virion is pleomorphic from spherical to filamentous. It contains eight negative sense, single-stranded RNA genomic segments, each coated with nucleoproteins (NPs) and bound to a heterotrimeric RNA polymerase complex of PA, PB1 and PB2 that forms as a viral ribonucleoprotein (vRNP). All 8 vRNPs are surrounded by a lipid bilayer (envelope). Whereas the inner layer of the envelope is attached to matrix 1 (M1) proteins bound to vRNPs and to nuclear export proteins (NEPs, formerly named NS2 proteins), the outer layer is spiked with HAs and NAs and channeled with matrix 2 (M2) proteins. (b) Diagram of an unfolded HA polypeptide. The signal peptide (SP) at the N-terminus is removed during the cotranslational translocation of a nascent HA into the ER. The synthesized single polypeptide HA is designed to be HA0 (inactive form) that carries HA1 and HA2 subunits. The HA1 subunit, in addition to being a major antigen, contains a receptor binding site, which binds specifically to a sialyl glycan as indicated, except for H17 and H18 viruses. A cleavage site (CS) is located at the end of the HA1 subunit. The HA1 CS usually contains a single basic aa (X-R/K) that can be cleaved extracellularly by tissue-specific expressed trypsin-like serine endoproteases. In contrast, the HA1 CS with additional basic amino acids (R/K-R) may be cleaved intracellularly by ubiquitous proteases (furin and PC5/6) in the trans-Golgi compartment recognizing the R-X-R/K-R motif or cleaved extracellularly by ubiquitous proteases (MSPL and TMPRSS13) in the plasma membrane recognizing the R/K-X-R/K-R motif, and the virus can thus cause systemic infection and is classified as a highly pathogenic (HP) virus. After the cleavage, the HA1 and HA2 subunits remain disulfide-linked between Cys (C, red) and Cys (C, green) of the two subunits, giving the fusion-active form of the HA. The HA2 subunit carries a hydrophobic fusion peptide (FP) at the N-terminus followed by heptad repeats 1 and 2 (HR1 and HR2). Upon activation at low pH, these 3 regions work cooperatively to fuse viral and cellular membranes together, releasing vRNPs into the host cell. The HA2 subunit also contains a transmembrane domain (TM) anchoring the viral membrane (envelope) and promoting full fusion. A hydrophilic cytoplasmic tail (CT) at the C-terminus of the HA2 subunit can also affect the fusion process. (c) Side view of a surface diagram of a trimeric HA. This representative viral HA is from pdb ID of 3ube, which showed a 2009 pandemic HA in complex with lactoseries tetrasaccharide c (LSTc, Neu5Acα2,6Galβ1,4GlcNAcβ1,3Galβ1,4Glc). Each monomer of the HA trimer is colored as follows: deep blue, HA1 and marine blue, HA2; green smudge, HA1 and lemon, HA2; raspberry, HA1 and red, HA2. The receptor binding site, which carries a part of the LSTc ligand (magenta stick, Neu5Ac; yellow stick, Gal; blue stick, GlcNAc; yellow stick, Gal), is located on each globular head, which is a part of HA1 on a stalk composed of the remaining part of HA1 and all HA2. |
| Date | |
| Source |
Sriwilaijaroen, N.; Suzuki, Y. Host Receptors of Influenza Viruses and Coronaviruses—Molecular Mechanisms of Recognition. Vaccines 2020, 8, 587. https://doi.org/10.3390/vaccines8040587 |
| Author | Nongluk Sriwilaijaroen and Yasuo Suzuki |
Licensing[edit]
This file is licensed under the Creative Commons Attribution 4.0 International license.
- You are free:
- to share – to copy, distribute and transmit the work
- to remix – to adapt the work
- Under the following conditions:
- attribution – You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 10:25, 31 January 2021 |  | 3,539 × 2,650 (2.37 MB) | Guest2625 (talk | contribs) | Uploaded a work by Nongluk Sriwilaijaroen and Yasuo Suzuki from Sriwilaijaroen, N.; Suzuki, Y. Host Receptors of Influenza Viruses and Coronaviruses—Molecular Mechanisms of Recognition. Vaccines 2020, 8, 587. https://doi.org/10.3390/vaccines8040587 with UploadWizard |
You cannot overwrite this file.
File usage on Commons
There are no pages that use this file.