File:Origins.gif
Origins.gif (450 × 303 pixels, file size: 13 KB, MIME type: image/gif, 0.2 s)
Captions
Captions
Summary[edit]
| DescriptionOrigins.gif |
English: Flow chart showing dry and wet deposition processes.
"Acid rain" is a broad term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. The precursors, or chemical forerunners, of acid rain formation result from both natural sources, such as volcanoes and decaying vegetation, and man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) resulting from fossil fuel combustion. In the United States, roughly 2/3 of all SO2 and 1/4 of all NOx come from electric power generation that relies on burning fossil fuels, like coal. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. The result is a mild solution of sulfuric acid and nitric acid. When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders, sometimes over hundreds of miles. Flow chart showing dry and wet deposition processes. If you have difficulty viewing this graphic, or need additional information, contact Cindy Walke, Web Manager, at 202-343-9194. Wet Deposition Wet deposition refers to acidic rain, fog, and snow. If the acid chemicals in the air are blown into areas where the weather is wet, the acids can fall to the ground in the form of rain, snow, fog, or mist. As this acidic water flows over and through the ground, it affects a variety of plants and animals. The strength of the effects depends on several factors, including how acidic the water is; the chemistry and buffering capacity of the soils involved; and the types of fish, trees, and other living things that rely on the water. Dry Deposition In areas where the weather is dry, the acid chemicals may become incorporated into dust or smoke and fall to the ground through dry deposition, sticking to the ground, buildings, homes, cars, and trees. Dry deposited gases and particles can be washed from these surfaces by rainstorms, leading to increased runoff. This runoff water makes the resulting mixture more acidic. About half of the acidity in the atmosphere falls back to earth through dry deposition. |
| Date | 12 February 2006 (original upload date) |
| Source | http://www3.epa.gov/acidrain/what/ |
| Author | Unknown authorUnknown author |

|
File:Origins of acid rain.svg is a vector version of this file. It should be used in place of this GIF file when not inferior.
File:Origins.gif → File:Origins of acid rain.svg
For more information, see Help:SVG.
|
Licensing[edit]
| Public domainPublic domainfalsefalse |
This image (or other media) is a work of an Environmental Protection Agency employee, taken or made as part of that person's official duties. As works of the U.S. federal government, all EPA images are in the public domain.
|
||
| العربية ∙ Deutsch ∙ English ∙ eesti ∙ italiano ∙ 日本語 ∙ македонски ∙ Nederlands ∙ polski ∙ português ∙ sicilianu ∙ slovenščina ∙ ไทย ∙ українська ∙ 简体中文 ∙ 繁體中文 ∙ +/− |
Original upload log[edit]
Transferred from en.wikipedia to Commons by Frokor.
- 2006-02-12 16:41 NHSavage 450×303×??? (13412 bytes) Downloaded from US EPA website: http://www.epa.gov/acidrain/origins.gif
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 16:58, 30 August 2008 | 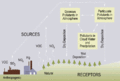 | 450 × 303 (13 KB) | File Upload Bot (Magnus Manske) (talk | contribs) | {{BotMoveToCommons|en.wikipedia}} {{Information |Description={{en|Downloaded from US EPA website: http://www.epa.gov/acidrain/images/origins.gif}} |Source=Transferred from [http://en.wikipedia.org en.wikipedia]; transferred to Commons by User:Frokor |
You cannot overwrite this file.
File usage on Commons
There are no pages that use this file.
File usage on other wikis
The following other wikis use this file:
- Usage on id.wikipedia.org
- Usage on min.wikipedia.org
- Usage on nn.wikipedia.org
- Usage on pt.wikipedia.org



